Module 5 : The Action Potential and its Propagation#
The action potential is a rapid, temporary change in the electrical potential difference (voltage) of a neuron’s membrane, which is critical for transmitting signals down an axon. It is the fundamental mechanism by which neurons communicate with one another and other cells. Another word used for the action potential is “spike”. In this module, we will study the phases, mechanisms and factors influencing the propagation of the action potential. [1]
During the action potential, the electrical potential moves from a negative resting potential to a positive value, and goes undershoot, and then again reaches back to resting potential. [1]
5.1 Voltage Gated Ion Channels#
In the previous lessons, we have studied about the passive leak ion channels which are unable to generate rapid changes in the membrane potential of a neuron, so here we need a new type of ion channel called Voltage Gated Ion Channels.
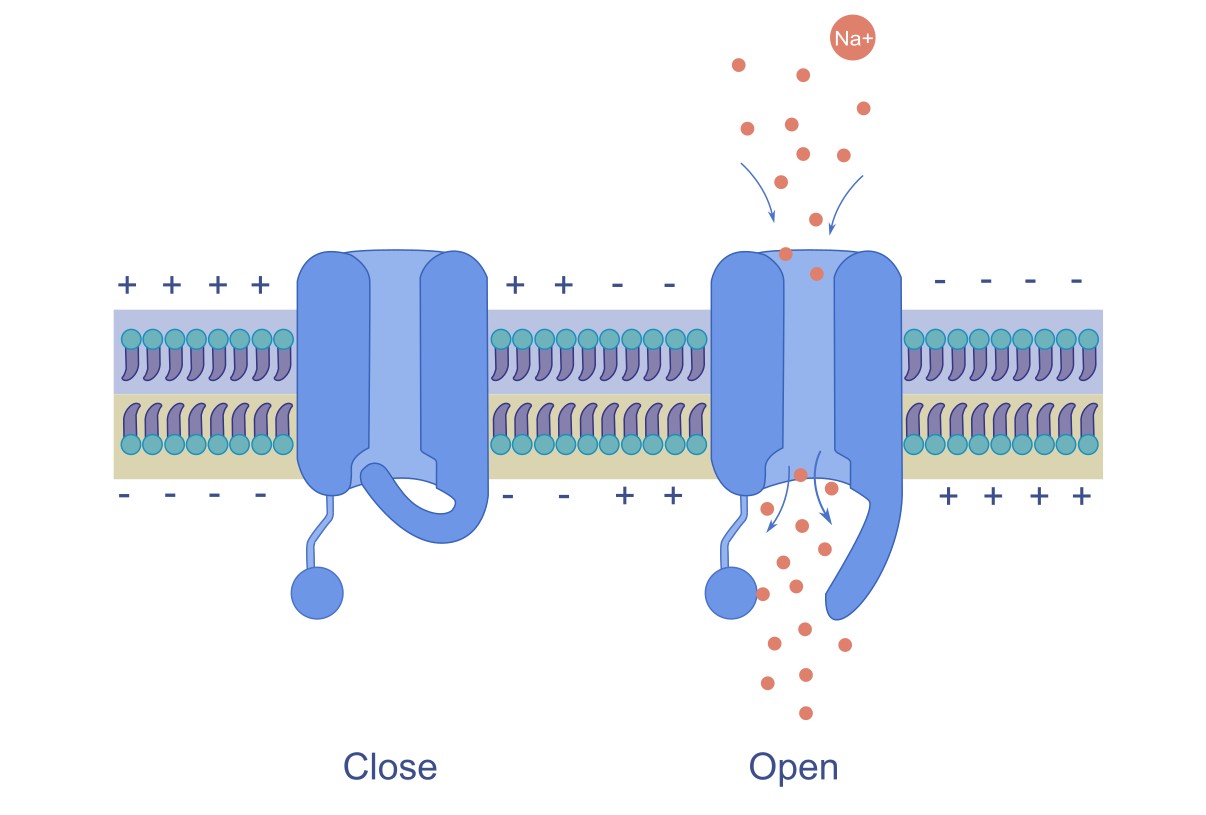
Voltage-gated channels are specialized membrane proteins that open or close in response to changes in the membrane potential. Their coordinated opening and closing allow neurons to transmit signals rapidly and effectively throughout the nervous system. The action potential arises from the activity of two primary voltage-gated ion channels: voltage-gated Na⁺ channels and K⁺ channels. These channels open and close at specific moments, generating ion flow, which leads to an action potential.
Note
Voltage gated ion channels open when the neuron’s membrane potential reaches a certain value called threshold value. [1]
5.2 Phases of the Action Potential#
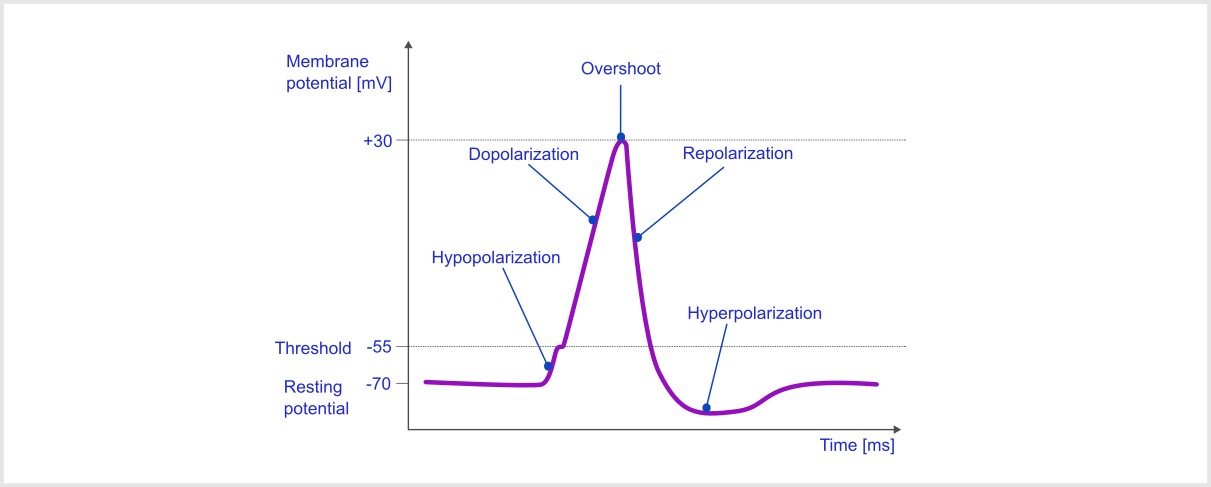
The action potential consists of several distinct phases, each characterized by specific changes in membrane potential and ion movement :
Resting Phase#
At the resting phase, the neuron has a resting potential of around -70mv, and the voltage-gated sodium and potassium channels are closed.
Depolarisation Phase (Rising Phase) [1]#
A stimulus causes the membrane potential to become less negative.
If depolarization reaches the threshold (around -55 mV), voltage-gated Na⁺ channels open.
Na⁺ ions rush into the neuron due to an electrochemical gradient, leading to a rapid rise in membrane potential, often reaching +30 mV.
Repolarization Phase (Falling Phase) [1]#
At the depolarization peak, the Na⁺ channels close.
After approx. 1ms, the sodium channel becomes inactive i.e., the channel gets blocked and the potassium channel becomes open.
K⁺ ions exit the neuron, causing the membrane potential to decrease and return to the resting level.
Note
Sodium channels open immediately, but potassium channels take some time . [1]
Hyperpolarization Phase (Undershoot) [1]#
The membrane potential temporarily becomes more negative than the resting potential (around -80 mV) due to the prolonged opening of K⁺ channels.
This phase makes the neuron less likely to fire another action potential immediately.
Return to Resting Phase [1]#
K⁺ channels close, and the sodium-potassium pump (Na⁺/K⁺ ATPase) restores the resting membrane potential.
The neuron is ready to fire another action potential when appropriately stimulated.
5.3 Action Potential Propagation#
Action potential propagation refers to the movement of an action potential (a rapid, transient electrical signal) along the membrane of an excitable cell, such as a neuron or muscle cell. The propagation of an action potential allows communication within the nervous system and muscle contraction. The process involves a sequence of events that ensures the signal is transmitted from the cell body to the axon terminal, where it can trigger neurotransmitter release or muscle contraction.
Action potentials typically move in one direction along an axon, from the cell body toward the axon terminal. This is due to the refractory period, which prevents backward propagation. [1]
Refractory Period#
The refractory period refers to the period of time following an action potential during which a neuron is unable to fire another action potential, or requires a stronger-than-normal stimulus to do so. This period ensures that action potentials propagate in one direction (without reversing course) and that the cell has enough time to reset its membrane potential to its resting state.
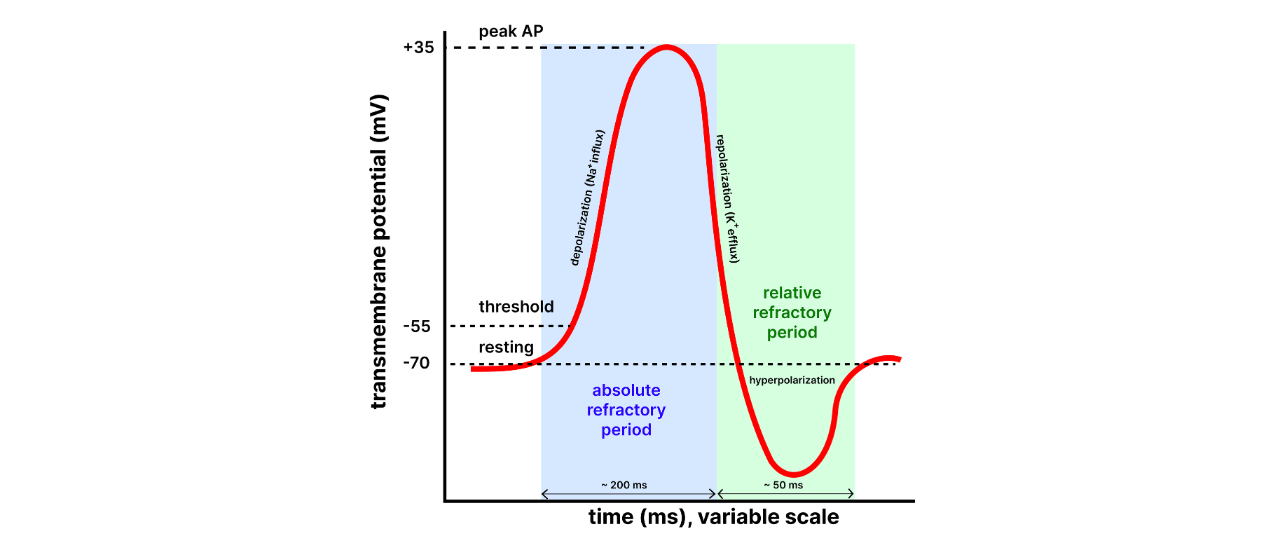
Types of Refractory Periods#
Absolute Refractory Period: [1]#
Definition: This is the period during and immediately after an action potential when the neuron is completely incapable of firing another action potential, no matter how strong the stimulus is.
Duration: It lasts from the beginning of depolarization to the end of repolarization (until the membrane potential returns to a sufficiently negative value). Cause: During the absolute refractory period, the voltage-gated Na⁺ channels are either open or inactivated, preventing any further depolarization. The inactivation gates of the Na⁺ channels are closed, meaning that no new action potential can be initiated until they reset.
Significance: This period ensures that the action potential travels in only one direction along the axon, as the region that has already undergone depolarization cannot be re-excited immediately. It also prevents the overlapping of action potentials.
Relative Refractory Period: [1]#
Definition: This is the period that follows the absolute refractory period, during which the neuron can generate another action potential, but only if the stimulus is stronger than normal.
Duration: The relative refractory period begins after repolarization, typically starting during the later stages of hyperpolarization, and it ends when the membrane potential returns to its resting level.
Cause: During this period, voltage-gated K⁺ channels are still open, causing the membrane potential to be more negative than usual (hyperpolarized). While some of the Na⁺ channels are back to their resting state and capable of reopening, not all of them are reset, so a stronger-than-usual stimulus is needed to overcome this state.
Significance: The relative refractory period allows for the possibility of a new action potential but prevents excessive firing, ensuring that neurons do not fire too frequently.
Factors affecting speed of propagation#
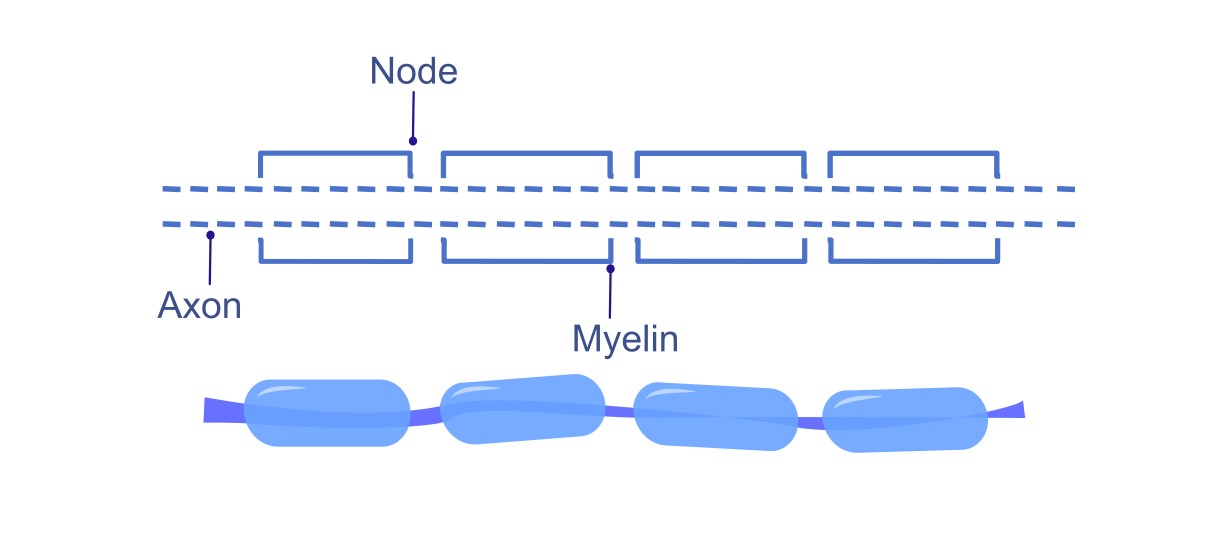
Myelination : [1]#
Myelin Sheath: Many axons are covered in myelin, a fatty substance that insulates the axon and increases the speed of action potential propagation.
Nodes of Ranvier: Action potentials jump between nodes of Ranvier (gaps in the myelin sheath) through a process known as saltatory conduction. This significantly speeds up the transmission of impulses compared to continuous conduction in unmyelinated axons.
Diameter of Axon : [1]#
The diameter of the axon plays a crucial role in determining the speed at which an action potential travels along it. Specifically, a larger axon diameter results in faster transmission of the action potential. This is because a wider axon offers less resistance to the flow of ions. As a result, sodium ions are able to move more rapidly, which facilitates the quicker regeneration of the action potential in the adjacent segments of the axon. Essentially, the larger the axon, the more efficiently it can conduct electrical signals due to reduced ion flow resistance.
Important Fact
When myelin is damaged, such as in demyelinating diseases like multiple sclerosis, the previously insulated areas of the axon become exposed. This leads to an increase in the capacitance of the exposed membrane, meaning it can store more charge. Consequently, this allows some of the electrical current to leak out of the axon, reducing the efficiency of signal transmission. As a result, the action potential that reaches these unmyelinated sections of the axon begins to weaken or decay, preventing it from being successfully propagated further along the axon. Essentially, the loss of myelin disrupts the normal flow of the electrical signal, leading to a failure in communication between nerve cells(i.e., action potential stops propagating).
Additional Info Section#
Tetrodotoxin (TTX) is a potent neurotoxin known for blocking nerve function, leading to paralysis and potentially death. It is found in certain animals, particularly pufferfish (fugu), but also in some other marine organisms, such as certain species of octopus, newts, and frogs.
Mechanism of Action:#
Tetrodotoxin works by selectively binding to voltage-gated sodium channels in the membranes of nerve cells, blocking sodium ions from entering the cells. This interferes with the action potential, which is critical for nerve signaling.
Blocking Sodium Channels:#
TTX binds to the outer pores of voltage-gated sodium channels, preventing the influx of sodium ions during depolarization. This blocks the generation of action potentials.
Effect on Nerve Function:#
The inability to generate action potentials prevents nerves from communicating with each other, leading to paralysis and respiratory failure.
Sources of Tetrodotoxin :#
Tetrodotoxin is produced by certain bacteria, not directly by the animals that carry it. These animals accumulate TTX through their diet, usually from consuming TTX-producing microorganisms like certain Vibrio species.
Despite its toxicity, many animals that carry TTX are not harmed by it due to adaptations in their sodium channels, which prevent TTX from binding effectively. Tetrodotoxin is extremely potent. As little as 2–3 mg of TTX is enough to kill a human. The mechanism by which it causes death is primarily through respiratory paralysis due to the blocking of nerve transmission.
Medical Research and Uses :#
Despite its toxicity, TTX has attracted attention in medical research for its potential as a painkiller and as a tool for studying sodium channel function. Pain Management: Due to its ability to block sodium channels and its potential to inhibit pain pathways without affecting other sensory functions, TTX has been investigated for use in localized pain treatment (e.g., in patients with chronic pain conditions like neuropathic pain or post-surgical pain). Sodium Channel Research: TTX is a valuable tool in neuroscience and pharmacology for studying the role of sodium channels in nerve function.
Fun Fact:#
Pufferfish is eaten for its delicate, subtle flavor and the tingling, numbing sensation that the TTX causes when eaten in small doses. [2]
References